Introduction:
The pharmaceutical industry is not just about manufacturing medications; it’s a direct connection to human life, where precision is paramount. Any error, even the slightest one, can have profound consequences. Recognizing this critical need for accuracy, Propix Technologies has emerged as a frontrunner in developing advanced vision inspection solutions for the pharmaceutical sector.
Precision from Pinholes detection on blisters to Enabling End to End Serialization Process:
Propix Technologies has been at the forefront of crafting solutions that leave no room for error. From pin-hole detection systems to complete end-to-end track and trace solutions, Propix ensures that every aspect of pharmaceutical production is flawless. What sets Propix apart is its unwavering commitment to meeting the ever-evolving stringent quality control paradigm in Pharma Industry
16 Years of Excellence:
With an impressive legacy spanning 16 years, Propix Technologies has successfully delivered a multitude of products and projects tailored to the unique demands of the pharmaceutical industry. While the Blister Inspection System, Code Inspection System, is a testament to their prowess in inspection systems, Propix goes beyond expectations. Their ‘PACKi Track and Trace’ system stands out as one of the most widely used Serialization system in the pharmaceutical industry, with installations in over 800 pharma packaging lines worldwide.
Unveiling Propix Serialization Systems:
Propix Serialization Systems caters to diverse packaging lines, from manual to fully automatic, seamlessly accommodating various formats like Cartons, Containers/Bottles, Vials, Pouches, and Special Packs. Fully compliant with country-specific regulations including USA-DSCSA, EU-FMD, Russia-CRPT, India-DGFT, Uzbekistan-Crypto, South Korea, UAE-Tatmeen, China-NMPA, Bahrain-NHRA-MVC, Turkey, Saudi Arabia-RSD, Australia-TGA, Indonesia-BADAN POM, and adaptable to new regulations, ensuring 21 CFR Part11 compliance.
Product Showcase:
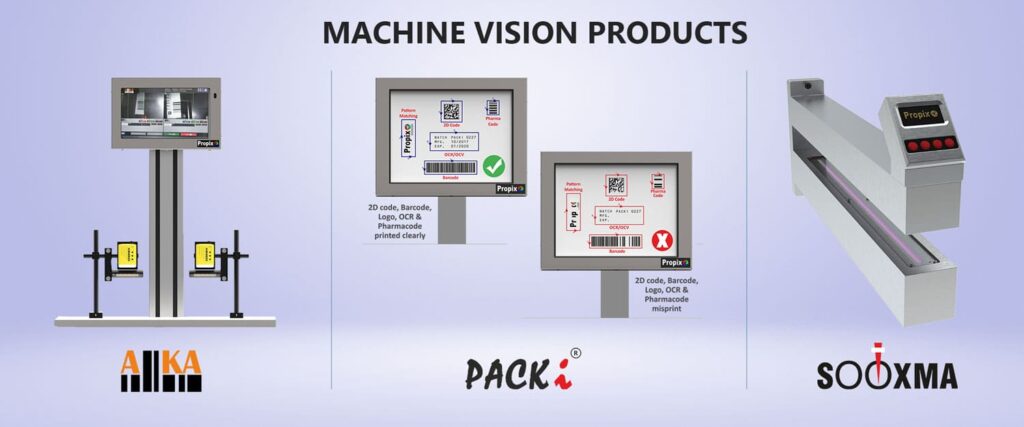
PACKi – Complete Vision Inspection System:
– Guarantees the highest level of online inspection of OCR / OCV/1D/2D Code/Pharma code
– Presence / absence on labels/cartons/pouches
Aiika – Code Inspection System:-
– Ensures precise validation and verification of all types of codes, minimizing the risk of errors and mix-ups
Sooxma – Pinhole Detection System:
– Detects punctures known as pinholes online on the aluminium foil with accuracy of 10 micron

Akshat – Serialization and Tamper-Evident Labelling System:
– Addresses EU FMD serialization requirements while ensuring the security of pharmaceutical packaging
Chatoor – Single camera based 360° 2D Code Decoding System:
– Addresses high speed DSCSA AGGREGATION requirements on bottles with smaller footprints
Vayu – Mono Carton Serialization Machine:
– Addresses high speed serialization for mono cartons for all types of regulatory compliances
Akash – Multi 2D code Scanning System:
– Ensures layer wise multi 2D code scanning system for bundles/ outer box/ case without camera movement
The Regulatory Landscape:
In response to the evolving regulatory landscape, Propix acknowledges and embraces the changes. The recent extension until 01.02.2025 for the implementation of the Track and Trace system for export consignments, ensuring the maintenance of the Parent-Child relationship in packaging levels, further underscores Propix’s commitment to compliance and adapting to industry standards.
Conclusion:
Propix Technologies stands as a beacon of precision in the pharmaceutical industry. With a rich history of delivering impeccable solutions and a commitment to meeting and exceeding regulatory requirements, Propix continues to redefine the standards for pharmaceutical safety. As the pharmaceutical landscape evolves, Propix remains at the forefront, pioneering innovative solutions that safeguard the integrity of medications and, by extension, human life.
